A ‘cage of cages’ is how scientists have described a brand new sort of porous materials, distinctive in its molecular construction, that may very well be used to lure carbon dioxide and one other, stronger greenhouse gasoline.
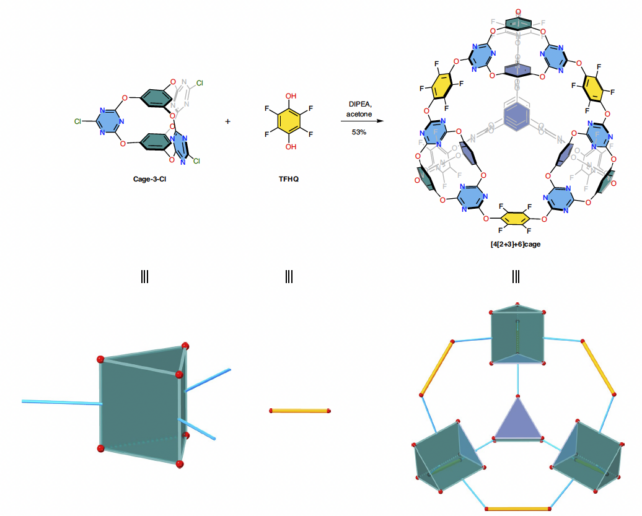
Synthesized within the lab by researchers within the UK and China, the fabric is made in two steps, with reactions assembling triangular prism constructing blocks into bigger, extra symmetrical tetrahedral cages – producing the primary molecular construction of its sort, the staff claims.
The ensuing materials, with its abundance of polar molecules, attracts and holds greenhouse gasses equivalent to carbon dioxide (CO2) with sturdy affinity. It additionally confirmed wonderful stability in water, which might be vital for its use in capturing carbon in industrial settings, from moist or humid gasoline streams.
“This is an exciting discovery,” says Marc Little, a supplies scientist at Heriot-Watt College in Edinburgh and senior creator of the research, “because we need new porous materials to help solve society’s biggest challenges, such as capturing and storing greenhouse gasses.”
Though not examined at scale, lab experiments confirmed the brand new cage-like materials additionally had a excessive uptake of sulfur hexafluoride (SF6), which in accordance with the Intergovernmental Panel on Local weather Change, is essentially the most potent greenhouse gasoline.
The place CO2 lingers within the environment for five–200 years, SF6 can grasp about for wherever between 800 to three,200 years. So though SF6 ranges within the environment are a lot decrease, its extraordinarily lengthy lifetime offers SF6 a worldwide warming potential of round 23,500 instances that of CO2 in comparison over 100 years.
Eradicating giant quantities of SF6 and CO2 from the environment, or stopping them from coming into it within the first place, is what we urgently must do to reign in local weather change.
Researchers estimate that we have to extract round 20 billion tons of CO2 every year to cancel out our carbon emissions which might be solely trending upwards.
To this point, carbon elimination methods are eradicating about 2 billion tons per 12 months, however that is largely timber and soils doing their factor. Solely about 0.1 p.c of carbon elimination, round 2.3 million tons per 12 months, is because of new applied sciences equivalent to direct air seize, which makes use of porous supplies to absorb CO2 from the air.
Researchers are busy devising new supplies to enhance direct air seize to make it extra environment friendly and fewer energy-intensive, and this new materials may very well be an alternative choice. However to avert the worst impacts of local weather change, we have to cut back greenhouse gasoline emissions quicker than these nascent applied sciences at present can.
Nonetheless, we have to throw all the pieces we are able to at this world drawback. Creating a cloth of such excessive structural complexity wasn’t straightforward although, even when the precursor molecules technically assemble themselves.
This technique is known as supramolecular self-assembly. It will possibly produce chemically interlocked constructions from less complicated constructing blocks, nevertheless it takes some fine-tuning as a result of “the best reaction conditions are often not intuitively obvious,” Little and colleagues clarify of their printed paper.
The extra advanced the ultimate molecule, the more durable it turns into to synthesize and extra molecular ‘scrambling’ may happen in these reactions.
To get a deal with on these in any other case invisible molecular interactions, the researchers used simulations to foretell how their starter molecules would assemble into this new sort of porous materials. They thought of the geometry of potential precursor molecules, and the chemical stability and rigidity of the ultimate product.
Apart from its potential to soak up greenhouse gasses, the researchers counsel their new materials is also used to take away different poisonous fumes from the air, equivalent to unstable natural compounds, which simply grow to be vapors or gasses from surfaces together with the within of recent automobiles.
“We see this study as an important step towards unlocking such applications in the future,” Little says.
The research has been printed in Nature Synthesis.