At the far finish of the periodic desk is a realm the place nothing is sort of appropriately. The weather right here, beginning at atomic quantity 104 (rutherfordium), have by no means been present in nature. In reality, they’d emphatically desire to not exist. Their nuclei, bursting with protons and neutrons, tear themselves aside by way of fission or radioactive decay inside instants of their creation.
These are the superheavy parts: after rutherfordium come dubnium, seaborgium, bohrium, and different oddities, all the best way as much as the heaviest ingredient ever created, oganesson, ingredient 118. People have solely ever made vanishingly small quantities of those parts. As of 2020, 18 years after the primary profitable creation of oganesson in a laboratory, scientists had reported making a complete of 5 atoms of it. Even when they may make way more, it could by no means be the form of stuff you could possibly maintain in your hand—oganesson is so radioactive that it could be much less matter, extra warmth.
Utilizing ultrafast, atom-at-a-time strategies, researchers are beginning to discover this unmapped area of the periodic desk and discovering it as fantastical as any medieval cartographer’s imaginings. Right here on the uncharted shoreline of chemistry, atoms have a bunch of bizarre properties, from pumpkin-shaped nuclei to electrons sure so tightly to the nucleus they’re topic to the principles of relativity, not in contrast to objects orbiting a black gap.
On supporting science journalism
If you happen to’re having fun with this text, take into account supporting our award-winning journalism by subscribing. By buying a subscription you might be serving to to make sure the way forward for impactful tales in regards to the discoveries and concepts shaping our world right now.
Their properties could reveal extra in regards to the primordial parts created in huge astrophysical phenomena comparable to supernovae and neutron star mergers. However greater than that, learning this unusual matter could assist scientists perceive the extra typical matter that happens naturally throughout us. As researchers get higher at pinning these atoms down and measuring them, they’re pushing the boundaries of the best way we manage matter within the first place.
“The periodic table is something fundamental,” says Witold Nazarewicz, a theoretical nuclear physicist and chief scientist on the Facility for Uncommon Isotope Beams at Michigan State College. “What are the limits of this concept? What are the limits of atomic physics? Where is the end of chemistry?”
Affixed to the wall in a concrete-block hall often known as Cave 1 in Lawrence Berkeley Nationwide Laboratory (LBNL), simply steps from one of many few devices on this planet that may create superheavy atoms, is a poster-size printout of a desk that organizes parts by nuclide, that means primarily based on the variety of protons and neutrons within the nucleus. This graph reveals all of the identified details about the nuclear construction and decay of the weather, in addition to of their isotopes—variations on parts with the identical variety of protons within the nucleus however completely different numbers of neutrons.
It’s a residing doc. There’s a typo within the title, and there are tears alongside the poster’s edges the place duct tape holds it to the wall. It’s been marked up with notations in Sharpie, added after the poster was printed in 2006. These notations are the atomic physics model of seafarers penciling in new islands as they sail, however on this case, the islands are isotopes of parts so heavy they are often seen solely in particle accelerators just like the one right here. In a discipline the place it could actually take every week to make only one atom of what you need, a document of progress is crucial.
“Everybody likes the handwritten part,” says Jacklyn Gates, who leads LBNL’s Heavy Factor Group. “If we were to print this out from 2023—”
“It’s not as fun,” chimes in Jennifer Pore, a employees scientist within the lab.
“It’s not as fun,” Gates agrees.
Gates is a nuclear chemist with a wry humorousness and a transparent fondness for the gear that she and her group have developed to synthesize superheavy parts. They create these parts by smashing standard-size atoms collectively in a 2.2-meter-wide cyclotron—a drum-shaped particle accelerator—in a lab perched on a hillside above town of Berkeley. Development on the cyclotron began in 1958, after the fallout from the primary nuclear bomb explosions started turning up within the type of new radioactive parts comparable to fermium (atomic quantity 100). A lot of the unique cyclotron persists right now; within the management room, silver dials that wouldn’t be misplaced in a chilly struggle–period thriller sit beside beige panels from the Eighties and blue banks of buttons from trendy updates.
The primary of the superheavies, rutherfordium, was synthesized right here in 1969. Rutherfordium, named after Ernest Rutherford, who helped to elucidate the construction of atoms, was additionally made a number of years prior by the Russian Joint Institute for Nuclear Analysis (JINR) in Dubna, the identical group that first created oganesson in 2002 (named after Yuri Oganessian, who led the group that created it). Starting within the late Nineteen Fifties, the competitors so as to add new parts acquired hotter than the ion beams used to make them. At the moment the vicious disputes over who synthesized what first, largely between the Berkeley lab and JINR, are remembered because the Transferium Wars.
By the Eighties Germany had joined the fray with its nuclear analysis institute, Gesellschaft für Schwerionenforschung (GSI), or the Society for Heavy Ion Analysis. The numbers ticked larger, with the three groups buying and selling off naming rights as much as copernicium (ingredient 112, named after Nicolaus Copernicus), found in 1996. Controversy continued to canine the superheavies; in 1999 researchers at LBNL introduced the invention of ingredient 116, now often known as livermorium after Lawrence Livermore Nationwide Laboratory, solely to retract that declare after discovering that certainly one of their scientists had fabricated proof. (JINR efficiently created livermorium in 2000.) In 2004 Japan’s Institute of Bodily and Chemical Analysis (RIKEN) synthesized ingredient 113, nihonium, after the Japanese phrase for “Japan.” Though ingredient 118 is the heaviest ingredient ever synthesized, probably the most not too long ago found is definitely 117, tennessine, which was introduced by JINR in 2010. The scientists behind the invention named it in tribute to the state of Tennessee, residence to a number of establishments that performed a job within the experiments.
“What are the limits of atomic physics? Where is the end of chemistry?”
—Witold Nazarewicz Michigan State College
The race to create ever heavier parts continues to at the present time, and never simply because the researchers who succeed get to call a brand new ingredient within the periodic desk. It’s additionally as a result of theorists predict that sure combos of protons and neutrons could land in an “island of stability” the place these parts will cease decaying instantly. “Some theories predict a year half-life, or 100 or 1,000 days,” says Hiromitsu Haba, a physicist and director of the Nuclear Chemistry Group at RIKEN, which is at present on the hunt for ingredient 119.
A half-life—the time it takes for about half of a substance’s atoms to decay—that lengthy could be sufficient for severe experimentation and even use in new applied sciences. For now, although, analysis into superheavies is concentrated on their elementary properties and what they will reveal about nuclear dynamics, not what they will do as supplies themselves. That doesn’t imply they received’t finally turn out to be helpful, nevertheless.
“Everything we’re doing right now … it doesn’t have practical applications,” Gates says. “But if you look at your cell phone and all the technology that went into that—that technology started back in the Bronze Age. People didn’t know it would result in these devices that we’re all glued to and utterly dependent on. So can superheavy elements be useful? Maybe not in my generation but maybe a generation or two down the road, when we have better technology and can make these things a little bit easier.”
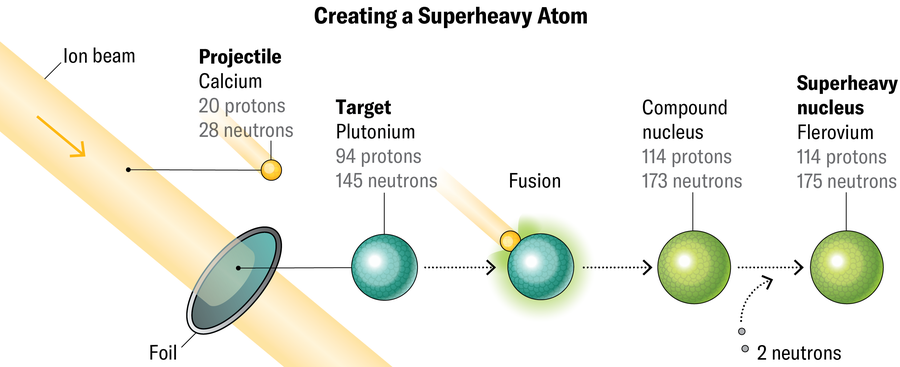
Making these parts is way from simple. Researchers do it by capturing a beam of heavy ions (on this case, giant atomic nuclei with out their electrons) at a goal materials within the hopes of overcoming the electrostatic repulsion between two positively charged nuclei and forcing them to fuse. At LBNL, the supply of the ion beam is a tool referred to as VENUS (for “versatile electron cyclotron resonance ion source for nuclear science”), which sits on the prime of the cyclotron behind fencing festooned with radiation warnings. Inside VENUS, a mixture of microwaves and robust magnetic fields strips electrons off a selected ingredient (typically calcium or argon in Gates’s experiments). The ensuing ions shoot down a pipeline into the cyclotron, which sweeps the ions round in a spiral, accelerating the beam.
Technicians within the management room use electrostatic forces to direct the beam out of the cyclotron and into devices within the “caves,” low corridors that come off the cyclotron like spokes. The caves include beam targets; the one in Cave 1 is a skinny steel foil in regards to the diameter of a salad plate. The targets rotate so the beam doesn’t hit any single spot for too lengthy. They will soften when bombarded with dashing ions, Gates says.
What the goal is product of relies on what number of protons the researchers need within the remaining product. For instance, to make flerovium (114 protons, named after Russian physicist Georgy Flerov, who based JINR), they should hit plutonium (94 protons) with calcium (20 protons). To make ingredient 118, oganesson, scientists beam calcium at californium (98 protons). The extra neutrons they will pack into the ion beam, the extra they will finally cram into the ultimate product, making even heavier isotopes.
More often than not the beam passes proper by the goal with none nuclear interactions. However with six trillion beam particles winging by the targets per second, an eventual nucleus-to-nucleus collision is inevitable. When circumstances are good, these pileups mash the nuclei collectively, creating a really short-term new superheavy atom transferring at almost 600,000 meters per second.
To decelerate these dashing heavyweights, the researchers use helium fuel and electrical fields to information the particles right into a entice for measurement. They will additionally pump in different gases to see what sorts of chemical reactions a superheavy ingredient will endure earlier than it decays. However that’s possible provided that the ingredient lasts lengthy sufficient, says Christoph E. Düllmann, head of the superheavy ingredient chemistry analysis group at GSI. To conduct and research chemical reactions, researchers require a component with a half-life of no less than half a second.
Scientists quantify superheavy parts and their response merchandise by measuring the vitality they provide off throughout alpha decay, the shedding of bundles of two protons and two neutrons. In a room referred to as the Shack at LBNL, researchers wait on tenterhooks for knowledge factors displaying them the place these alpha-decay particles land contained in the detector; their journey reveals details about the composition of the unique atoms and any reactions they’ve undergone. It’s onerous to think about that chemistry bodily occurring, Pore says: “It almost feels like it exists somewhere else.”
The heaviest ingredient that researchers have studied chemically is flerovium (114)—the heaviest one that may be created within the portions and with the length wanted for chemical experiments. Scientists can produce flerovium at a price of about three atoms a day, Düllmann says. “A typical experiment needs about one month of total run time,” he says. “Not every atom that is produced will reach your chemistry setup, and not every atom that reaches your chemistry setup will be detected in the end.”
Just a few atoms can reveal so much, nevertheless. Earlier than flerovium was synthesized, some theories predicted that it would act like a noble fuel—inert and nonreactive—and others advised it would act like a steel, particularly, mercury. Experiments on the ingredient revealed in 2022 within the journal Frontiers in Chemistry confirmed one thing weirder. At room temperature, flerovium kinds a powerful bond with gold, very in contrast to a noble fuel. It additionally bonds with gold at liquid-nitrogen temperatures (–196 levels Celsius). Oddly, although, at temperatures between these two, the ingredient doesn’t react.
Oganesson is grouped within the periodic desk with the noble gases, however researchers suppose it’s neither noble nor a fuel. It’s in all probability a stable at room temperature, in accordance with analysis revealed in 2020 in Angewandte Chemie, and transitions to liquid round 52 levels C. There are numerous such examples, says Peter Schwerdtfeger, a theoretical chemist at Massey College in New Zealand and senior writer of the 2020 paper.
The explanation for these unusual traits has to do with the electrons. Electrons orbit nuclei at sure vitality ranges often known as shells, every of which may maintain a selected variety of electrons. Electrons in outer shells—the place there might not be sufficient electrons to utterly fill the shell—are chargeable for forging chemical bonds with different atoms. Every shell ostensibly represents a selected distance from the nucleus, though the precise path of an electron’s orbit in that shell (referred to as an orbital) is commonly removed from a easy circle and may look extra like a dumbbell, doughnut, teardrop, or different configuration. (Based on quantum mechanics, these outlines merely signify the locations the place an electron is more likely to be discovered if pinned down by an precise measurement. In any other case, electrons largely exist in a haze of chance someplace across the nucleus.)
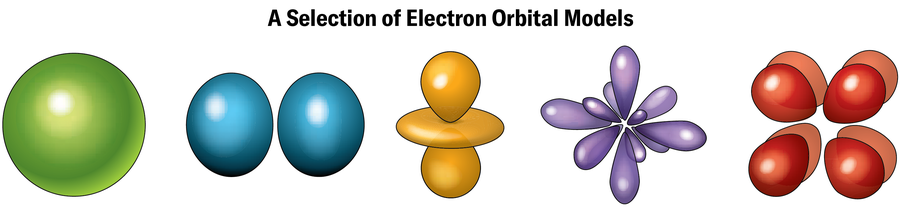
As a nucleus will get heavier, electrons close to it really feel an excessive pull from the glut of optimistic costs there, drawing them in nearer and lowering the area they’ve to maneuver round in. Due to the uncertainty precept, which states {that a} particle’s place and velocity can’t be identified exactly on the identical time, this discount within the electrons’ elbow room means their velocity should enhance by way of a form of seesawing of elementary bodily legal guidelines. Quickly the electrons are touring at almost the velocity of sunshine. As Einstein’s common principle of relativity suggests, objects transferring this quick acquire mass and get bizarre. Specifically, the orbits of electrons within the lowest-energy states—the innermost shells—round a superheavy nucleus are inclined to contract, making a better density of electrons nearer to the nucleus, Schwerdtfeger says. These modifications are often known as relativistic results.
These results present up even in naturally occurring parts of the periodic desk. Gold is yellowish as a result of relativistic results shrink the hole between two of its electron shells, barely shifting the wavelengths of sunshine that the ingredient absorbs and displays. But relativistic results don’t often play an enormous position within the chemical conduct of most gentle parts. That’s why the order of parts within the periodic desk relies on the variety of protons in every ingredient’s nucleus. This association serves to group collectively substances with comparable chemical properties, that are decided primarily by the variety of electrons in outer shells which can be accessible for chemical bonds.
“The periodic table is supposed to tell you what the chemical trends are,” LBNL’s Pore says. For heavier parts, during which relativistic results begin to rule, that’s not essentially true. In analysis revealed in 2018 within the journal Bodily Evaluate Letters, Schwerdtfeger and his colleagues discovered that due to relativistic results, oganesson’s electron cloud seems to be like an enormous, fuzzy smear with no main distinction between the shells.
Even exterior superheavy territory, chemists debate the location of sure parts within the periodic desk. Since 2015 a working group on the Worldwide Union of Pure and Utilized Chemistry has been refereeing a debate over which parts ought to go within the third column of the desk: lanthanum and actinium (parts 57 and 89) or lutetium and lawrencium (71 and 103). The controversy facilities on misbehaving electrons: due to relativistic results, the outermost electrons orbiting these parts aren’t the place they need to be in accordance with the periodic desk. After 9 years of official consideration, there may be nonetheless no consensus on the way to group these parts. Such issues solely turn out to be extra urgent on the heavier finish of the desk. “We’re trying to probe where that organization begins to break down and where the periodic table begins to stop being useful,” Gates says.
Together with a window into the boundaries of chemistry, the dance of electrons can present a peek into the dynamics of the nucleus on the extremes. In a nucleus groaning with protons and neutrons, interactions between these particles typically warp the form into one thing aside from the stereotypical sphere you’ll see in diagrams of atoms. Many of the superheavy parts which have been probed up to now have rectangular nuclei formed like footballs, says Michael Block, a physicist at GSI. Theoretically, heavier ones that haven’t been synthesized but may need nuclei formed like alien craft and even bubbles, with empty or low-density spots proper within the heart. Scientists “see” these shapes by measuring minuscule modifications in electron orbits, that are affected by the association of the optimistic costs within the nucleus. “This allows us to tell what the size of the nucleus is and what the shape of the nucleus is,” Block says.
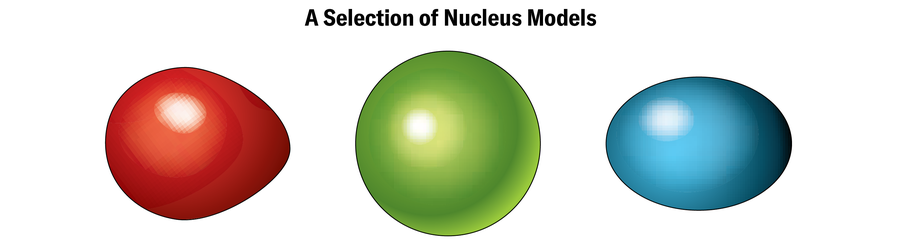
The structure of the nucleus holds the important thing as to if anybody will ever have the ability to synthesize a superheavy ingredient that sticks round. Sure numbers of protons and neutrons (collectively dubbed nucleons) are often known as magic numbers as a result of nuclei with these numbers can maintain collectively significantly effectively. Like electrons, nucleons occupy shells, and these magic numbers signify the tallies wanted to fill nucleonic shells utterly. The island of stability that researchers hope to seek out in a but undiscovered superheavy ingredient or isotope could be the results of “double magic”—theoretically perfect numbers of each protons and neutrons.
Whether or not such a factor exists is an open query as a result of heavy nuclei would possibly tear themselves aside reasonably than tolerating the required numbers of nucleons. “Fission is the killer,” M.S.U.’s Nazarewicz observes.
In contrast to the (comparatively) gradual whittling down of a nucleus by alpha decay, nuclear fission is a sudden and utter dissolution. Totally different fashions yield completely different predictions about what number of particles could be packed right into a nucleus earlier than fission turns into inevitable, Nazarewicz says. Theorists try to find out this restrict to know how giant nuclei can actually get.
There’s an attention-grabbing liminal area on the edges of what nuclei can bear, Nazarewicz notes. To be declared a component, a nucleus should survive for no less than 10–14 second, the time it takes for electrons to glom on and kind an atom. However in principle, nuclear lifetimes could be as quick as 10–21 second. On this infinitesimal hole, you would possibly discover nuclei with out electron clouds, incapable of chemistry, he says.
“The periodic table breaks with the heaviest elements already,” Nazarewicz says. The query is, The place do you break chemistry altogether? Another option to perceive superheavy parts is to search for them in area. The weather heavier than iron (atomic quantity 26) kind in nature by a course of referred to as fast neutron seize, which regularly happens in cataclysmic occasions comparable to a collision of two neutron stars.
If superheavies have ever arisen naturally within the universe, they had been made by this course of, too, says Gabriel Martínez-Pinedo, an astrophysicist at GSI. In fast neutron seize, also referred to as the r-process, a seed nucleus grabs free close by neutrons, rapidly taking over the mass to make heavy isotopes. This should occur in an setting with ample neutrons roaming freely, which is why neutron star mergers are opportune spots.
In 2017 scientists noticed a neutron star merger for the primary time by detecting gravitational waves created by the interplay. “That was the very first confirmation that, indeed, the r-process happens during the merger of two neutron stars,” Martínez-Pinedo says. Researchers detected isotopes of lanthanide parts (atomic numbers 57 to 71) in that merger however, as they reported in Nature on the time, couldn’t slim down the precise parts current. Detecting any superheavy parts shall be even trickier as a result of researchers might want to know which distinctive wavelengths of sunshine these parts emit and take up and choose them out of what Martínez-Pinedo calls the “complicated soup of elements” that emerges from certainly one of these occasions.
In December 2023, nevertheless, astronomers reported within the journal Science that there are extra quantities of a number of lighter parts—ruthenium, rhodium, palladium and silver—in some stars. These parts could also be overrepresented as a result of they’re the results of heavy or superheavy parts breaking up by way of fission. The findings trace that nuclei with as many as 260 protons and neutrons would possibly kind by way of the r-process.
Even when superheavy parts created in neutron star mergers had been to decay away rapidly, figuring out they existed would assist scientists write a historical past of matter within the universe, Martínez-Pinedo says. New observatories such because the James Webb Area Telescope and the upcoming Vera C. Rubin Observatory in Chile ought to make it attainable to see different cosmic occasions able to creating superheavy parts. “And there will be new gravitational-wave detectors that will allow us to see much larger distances and with higher precision,” he provides.
On the Facility for Uncommon Isotope Beams in Michigan, a brand new high-energy beam guarantees to offer additional insights into the r-process by packing extra neutrons into isotopes than ever earlier than attainable. These will not be new superheavies however beefed-up variations of lighter parts. In February researchers reported within the journal Bodily Evaluate Letters that that they had created heavy isotopes of thulium, ytterbium and lutetium utilizing only one 270th of their beams’ final deliberate energy output. At larger energy ranges they need to have the ability to make the sorts of isotopes that finally decay into heavier steady metals comparable to gold. “This may provide a pathway to some of the interesting isotopes for astrophysics,” says Brad Sherrill, a physicist at M.S.U. and a co-author of that research.
In the meantime different scientists world wide are additionally seeking to amp up their ion beams and targets to push previous ingredient 118. As well as, they’re growing the precision with which they will seize and measure these parts. Researchers on the Facility for Uncommon Isotope Beams plan to enhance their capacity to distinguish between particles by an element of 10. GSI will quickly have a next-generation accelerator for superheavy synthesis. And at LBNL, Gates and her group are putting in devices to take higher-precision measurements of the mass of single atoms.
These new instruments ought to additional reveal the contours of chemistry on the extremes. “When we do superheavy chemistry,” Massey’s Schwerdtfeger says, “we see surprises all over the place.”