The sliding filament principle is a elementary idea in muscle physiology that explains how muscle groups contract to generate power. This principle relies on the interactions between two varieties of protein filaments—actin (skinny filaments) and myosin (thick filaments)—inside the muscle fibers. By understanding this principle, we acquire perception into how muscle groups perform at a molecular degree to provide motion and power.
Construction of Skeletal Muscle
Skeletal muscle fibers, also called myofibers, are lengthy, cylindrical cells which are multinucleated as a result of fusion of precursor muscle cells throughout growth. These fibers are specialised for contraction and include a number of key buildings:
- Sarcoplasmic Reticulum (SR): A specialised sort of clean endoplasmic reticulum that shops and releases calcium ions (Ca²⁺), that are essential for muscle contraction.
- Myofibrils: These are the contractile components of the muscle fiber, consisting of thick myosin filaments and skinny actin filaments organized in a repeating sample alongside the size of the muscle fiber.
- T-Tubules (Transverse Tubules): Extensions of the sarcolemma (muscle cell membrane) that penetrate into the muscle fiber, permitting the motion potential to achieve the SR.
- Mitochondria: Quite a few in muscle fibers to offer the ATP wanted for contraction.
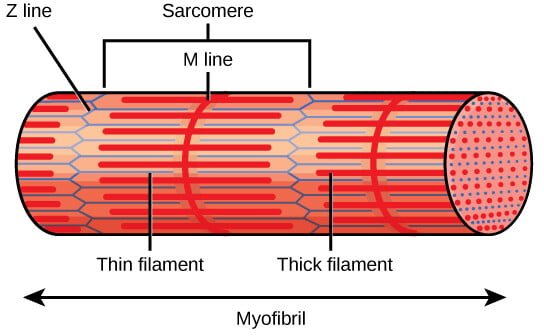
Construction of the Sarcomere
A sarcomere is characterised by its extremely organized construction, which will be noticed underneath a light-weight microscope as a repeating unit of alternating darkish and lightweight bands. The important thing structural components of the sarcomere embrace:
- Z Discs (Z Traces): The Z discs outline the boundaries of every sarcomere and anchor the skinny filaments of actin. They’re situated at each ends of the sarcomere and play an important position in sustaining the structural integrity of the sarcomere. The Z disc additionally serves as an attachment website for titin, a big protein that contributes to sarcomere elasticity.
- A Band: The A band is the area of the sarcomere that comprises your entire size of the thick filaments (myosin). It seems darker underneath the microscope as a result of overlap of myosin with actin filaments. The A band doesn’t change in size throughout muscle contraction, because it represents the size of the myosin filaments, which don’t shorten however relatively slide previous the actin filaments.
- I Band: The I band is the lighter area of the sarcomere that comprises solely skinny filaments (actin) and extends from the top of 1 A band to the start of the subsequent A band. The I band shortens throughout muscle contraction because the actin filaments slide inward, overlapping extra with the myosin filaments.
- H Zone: The H zone is a central area inside the A band the place solely thick filaments are current, with no overlap from skinny filaments. It seems lighter than the remainder of the A band. Throughout contraction, the H zone narrows because the actin filaments slide into the area, lowering the space between adjoining myosin filaments.
- M Line: The M line is a structural protein meeting situated within the middle of the sarcomere, inside the A band. It helps anchor the thick filaments and maintains their alignment throughout contraction. The M line consists of myomesin and different proteins that stabilize the myosin filaments.
Myosin: Construction, Synthesis, Classification, and Capabilities – The Science Notes
Actin: Construction, Perform, and Dynamics – The Science Notes
The Sliding Filament Idea
The sliding filament principle, initially proposed by A. F. Huxley and R. Niedergerke and later expanded by H. E. Huxley and J. Hanson, explains how muscle contraction happens. In accordance with the idea:
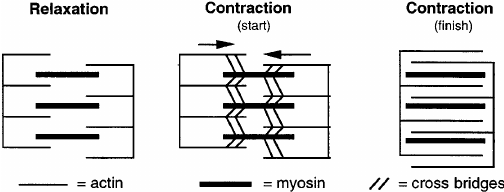
- Sarcomere Construction Throughout Contraction: As a muscle contracts, the A band, which comprises myosin filaments, stays the identical size, whereas the I band, which comprises actin filaments, shortens. This shortening is as a result of sliding of actin filaments previous myosin filaments.
- Actin-Myosin Interplay: Myosin heads bind to actin filaments, forming cross-bridges. These cross-bridges endure an influence stroke powered by ATP hydrolysis, pulling the actin filaments in direction of the middle of the sarcomere and inflicting contraction.
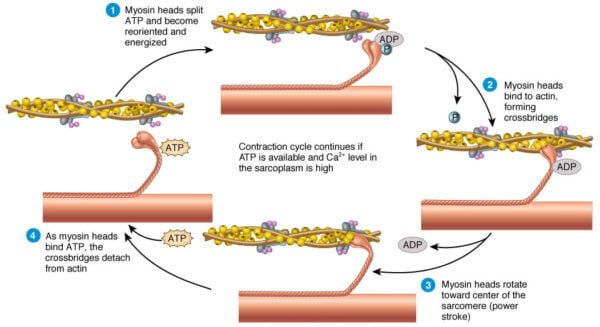
Steps of Sliding Filament Idea
In accordance with the sliding filament principle, muscle contraction happens by means of the sliding of actin and myosin filaments previous each other, ensuing within the shortening of the sarcomere with out altering the size of the filaments themselves. The method entails a number of key steps:
1. Motion Potential and Calcium Launch:
- An motion potential from a motor neuron arrives on the neuromuscular junction, inflicting the discharge of acetylcholine (ACh).
- ACh binds to receptors on the sarcolemma, resulting in depolarization and the initiation of an motion potential that travels alongside the T-tubules.
- The motion potential stimulates the SR to launch Ca²⁺ into the sarcoplasm.
2. Binding of Calcium to Troponin:
- Calcium ions bind to troponin C, a part of the troponin advanced related to the skinny filaments.
- This binding causes a conformational change within the troponin-tropomyosin advanced, shifting tropomyosin away from the actin-binding websites.
3. Cross-Bridge Formation:
With the binding websites uncovered, the high-energy myosin heads connect to actin, forming cross-bridges.
ATP sure to myosin is hydrolyzed to ADP and inorganic phosphate, offering vitality for the ability stroke.
4. Energy Stroke and Filament Sliding:
- In the course of the energy stroke, the myosin head pivots, pulling the actin filaments towards the M line and shortening the sarcomere.
- The discharge of ADP and phosphate from the myosin head is adopted by the binding of a brand new ATP molecule, which causes the detachment of the myosin head from actin.
5. Reactivation of Myosin Heads:
- ATP is hydrolyzed to ADP and phosphate, which re-energizes the myosin head, making ready it for the subsequent cycle of binding and pulling.
- The cycle repeats so long as calcium ions stay current within the sarcoplasm.
6. Muscle Rest:
- When the motion potential ceases, calcium ions are actively transported again into the SR.
- The lower in calcium focus causes tropomyosin to cowl the actin-binding websites once more, stopping additional cross-bridge formation and leading to muscle leisure.
Regulation of Muscle Contraction
Muscle contraction is tightly regulated by a number of components, together with calcium ions and ATP:
- Calcium’s Function: Calcium ions play a pivotal position in muscle contraction by binding to troponin, a regulatory protein related to actin filaments. Within the absence of calcium, tropomyosin covers the myosin-binding websites on actin, stopping cross-bridge formation. When calcium ranges improve, troponin undergoes a conformational change that strikes tropomyosin away from the binding websites, permitting myosin heads to connect to actin.
- ATP’s Function: ATP is important for muscle contraction because it offers the vitality wanted for the ability stroke and the detachment of myosin from actin. ATP hydrolysis additionally permits the myosin head to return to its cocked place. With out ample ATP, muscle groups can not chill out and stay in a state of contraction, resulting in circumstances reminiscent of rigor mortis.
Detailed Mechanisms and Observations
- Molecular Mechanisms: The detailed molecular mechanisms of the sliding filament principle have been elucidated by means of numerous research. For instance, high-resolution microscopy and X-ray diffraction research have offered insights into the structural adjustments that happen throughout muscle contraction. These research have confirmed that the sliding of actin filaments previous myosin is accountable for sarcomere shortening.
- Protein Buildings: The structural particulars of the myosin and actin filaments have been additional explored by means of strategies reminiscent of cryo-electron tomography and atomic power microscopy. These research have revealed the exact preparations of actin and myosin, in addition to the conformational adjustments that happen throughout the cross-bridge cycle.
Proof Supporting the Idea
A number of observations help the sliding filament principle:
- Sarcomere Shortening: Throughout muscle contraction, the space between Z-discs decreases, the H zone narrows, and the I band shortens, whereas the A band stays unchanged.
- Microscopic Research: Electron microscopy reveals that the actin and myosin filaments don’t change size however slide previous one another.
Conclusion
The sliding filament principle has profoundly formed our understanding of muscle contraction, revealing the intricate molecular interactions that allow muscle fibers to shorten and generate power. By elucidating the roles of actin, myosin, ATP, and calcium, scientists have gained helpful insights into muscle perform and regulation. Regardless of its complete nature, ongoing analysis continues to refine our understanding and deal with unresolved questions. The continued exploration of muscle physiology guarantees to uncover new insights and purposes, advancing our data of this elementary physiological course of.
References and Additional Studying
- Huxley, A. F., & Niedergerke, R. (1954). Structural adjustments in muscle throughout contraction: Interference microscopy of residing muscle fibres. Nature, 173, 971-973.
- Huxley, H. E., & Hanson, J. (1954). Modifications within the cross-striations of muscle throughout contraction and stretch and their structural interpretation. Nature, 173, 973-976.
- Spudich, J. A. (2001). The myosin swinging cross-bridge mannequin. Nature Evaluations Molecular Cell Biology, 2, 387-392.
- Lehman, W., Craig, R., & Vibert, P. (1994). Ca2+-induced tropomyosin motion in Limulus skinny filaments revealed by three-dimensional reconstruction. Nature, 368, 65-67.
- Goody, R. S. (2003). The lacking hyperlink within the muscle cross-bridge cycle. Nature Structural Biology, 10, 773-775.