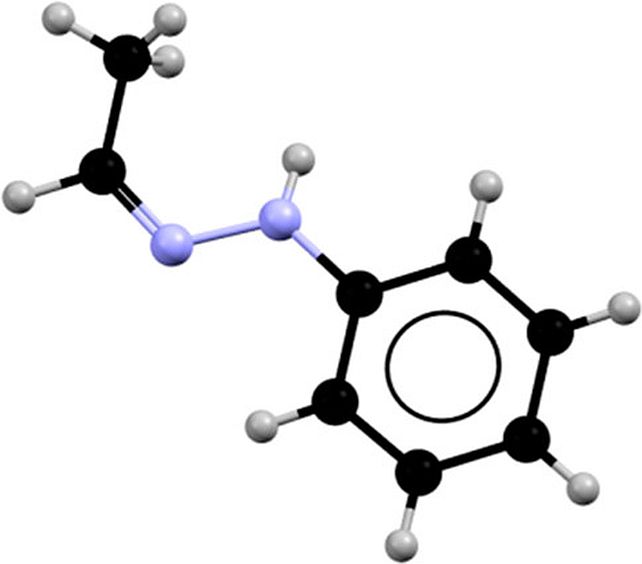
In 1896, German chemist Emil Fischer famous one thing very unusual a couple of molecule named acetaldehyde phenylhydrazone. Equivalent batches of the crystalline compound appeared to have wildly totally different melting factors.
Some batches, he discovered, melted at temperatures of round 65 levels Celsius (149 Fahrenheit). Others at 100 levels Celsius.
It was, in a phrase, totally weird. No different substance was identified to behave this fashion. Nor ought to it. In response to the legal guidelines of thermodynamics that describe the way in which the bodily world behaves, such a outcome ought to be inconceivable.
Scientists have been stumped. They rushed to see if Fischer had made a mistake. Think about their consternation after they have been capable of replicate his observations.
Greater than 120 years after Fischer’s authentic discovery, in 2019, a global crew of researchers led by chemist Terry Threfall of the College of Southampton within the UK lastly discovered and printed the reply. Fischer (who went on to win a 1902 Nobel prize for different work, so he was clearly no quack) had noticed one thing actual; however not, as it could prove, something that may break thermodynamics.
The offender? A completely miniscule contamination, so small that it’s all however undetectable. When acetaldehyde phenylhydrazone melts, it turns into certainly one of two liquids, based mostly on whether or not the compound has been uncovered to a base or an acid. The previous seems on the larger melting level; and the latter on the decrease.
“It is just exceedingly satisfying to be able to understand such an ancient puzzle, especially one which baffled such an eminent scientist who became a Nobel Prize winner,” Threlfall stated.
“The observation of such behavior will be exceedingly rare because it depends on the molecules in the crystal and in the liquid having different geometries, which is unusual. Furthermore, it depends also on the conversion by acid being both possible and rapid.”
The compound is made by dissolving strong acetaldehyde and including each liquid phenylhydrazine and aqueous ethanol, and chilling till the combination freezes and kinds strong crystals. To then uncover the melting level of the newly shaped acetaldehyde phenylhydrazone, you need to re-melt it.
That is the place the issues emerged. To know why acetaldehyde phenylhydrazone melts at two distinct temperatures, the researchers first investigated its strong type. However probably the most innovative probes failed to show up a solution.
All analyses, carried out by Threlfall’s crew and different current efforts, did not discover a single distinction between acetaldehyde phenylhydrazone samples that melted on the decrease temperature, and samples that melted on the larger. These methods included X-ray diffraction, nuclear magnetic resonance, and IR spectroscopy. So far as scientists may inform, the crystals have been similar.
The subsequent step was to analyze the liquid the crystals grew to become after melting.
And there, the researchers bought a outcome. There was a refined, and momentary, however distinct distinction. Though the compounds had the identical molecular components, the construction of the preliminary soften was barely totally different, relying on the temperature.
The compound accommodates a methyl group that is ready to have two distinct configurations, often called the Z isomer and the E isomer.
In its strong part, the fabric nearly completely consists of the Z isomer.
Probably the most steady liquid part is a mixture of about one-third Z isomer to two-thirds E isomer. The decrease of the 2 melting factors instantly produces the Z and E combine, whereas the upper melting level is totally Z, earlier than switching to half E.
A clue was given in a 1905 paper, which identified that acetaldehyde phenylhydrazone was extraordinarily delicate to acid. Threlfall and his crew tried exposing their samples to vapors of acid and ammonia. They usually discovered that publicity to only a tiny bit of 1 or the opposite may reliably affect the compound’s melting level. The acid acts as a catalyst to hurry the shift from the Z to E isomer, decreasing the melting level within the course of.
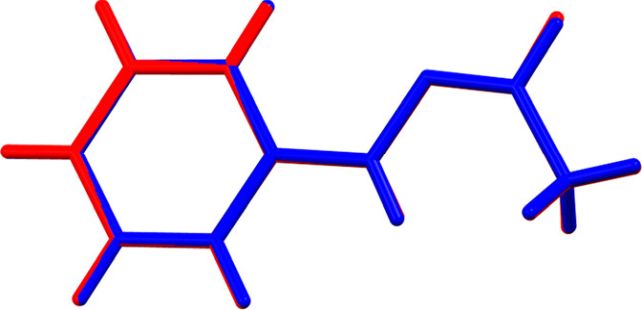
“If an element or compound can exist in two or more distinct crystalline forms, then each form will have different Gibbs energies and melt at its own distinct temperature,” stated chemist Simon Coles of the College of Southampton.
“In this case, the molecules of the crystal are in the cis geometry – of groups pointing towards each other – and melt to an identical geometry in the absence of acid at 100 degrees Celsius. However, in the presence of even a trace of acid, the molecules convert on melting to the trans geometry of groups pointing away from each other. This liquid has a smaller Gibbs energy and is more stable, so the melting point becomes 65 degrees Celsius.”
It is much like the impact salt has on water: including salt to a pot of water raises the freezing and boiling factors. The place it takes a number of salt to invoke a major change to water’s part transitions, it takes so little acid to change acetaldehyde phenylhydrazone that it took greater than a century – and Threlfall and his colleagues a decade – to determine it out.
This analysis is an actual testomony to human curiosity and tenacity. And it offers us hope for the long run. What number of extra mysteries will probably be solved within the years stretching right into a glittering way forward for discovery?
The analysis was printed in 2019 in Crystal Development & Design.